The importance of otoliths in fisheries biology
One of the biggest difficulties in fisheries research and management is that it is challenging to directly observe fish over the entire course of their life cycle. It is much easier to count sheep in a pasture than it is to count fish underwater. One of the most useful tools scientists use to study fish are otoliths.
Otoliths (Greek for “ear-stone”) are calcium carbonate structures found beneath the brain of most fish that aid in balance and hearing (Figure 1). The otoliths are not attached to the skull or any other bone, they float in fluid-filled sacs in the inner ear. Most fish have three pairs of otoliths: sagittae (the largest pair), lapilli, and asterisci. Only cartilaginous fishes (sharks, rays and chimaeras) and jawless fishes (lampreys and hagfish) do not have otoliths.
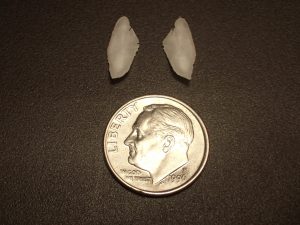
Figure 1. A pair of sagittal otoliths from a striped bass (Morone saxatilis) with a dime for scale. Photo by Steve Luell.
The size and shape of otoliths vary by species. Fish that live on structured habitat and vocalize during mating, such as black drum (Pogonias cromis) and Atlantic croaker (Micropogonias undulatus), have very large otoliths. Species that are continuously swimming, such as bluefin tuna (Thunnus thynnus) and alewives (Alosa pseudoharengus; Figure 2) have much smaller otoliths, possibly to limit excessive movements of the otoliths in the inner ear while the fish swims. Due to their distinct shapes and sizes, researchers can identify otoliths even when the rest of the fish’s body is absent. For example, some scientists analyze otoliths that are recovered from bird and seal excrement to learn about the dietary preferences of these predators.

Figure 2. A pair of sagittal otoliths from an alewife with a dime for scale. Photo by Steve Luell.
Otoliths continuously accrete calcium carbonate throughout the fish’s life. The calcium carbonate that is used to form the otoliths comes from the water and the food eaten by the fish. This process is influenced by the fish’s metabolism. During the winter, the otolith forms a dense, opaque layer due to slowed metabolism. In the warmer months when the metabolic rate is higher, the layer that is formed is less dense and translucent. The opaque bands that are formed represent yearly growth rings. These are used by fisheries scientists to estimate the fish’s age, just like counting the rings on a tree. By measuring length and weight in addition to age, scientists can also estimate growth. For younger fish under one year of age (known as “young-of-the-year (YOY)”), the otoliths form daily growth increments due to differences in feeding rates and water temperatures between day and night. By counting the daily growth rings, scientists can find the exact date that the young fish hatched from its egg. This is especially useful for scientists studying the growth rates of larval fish.
Otoliths can be used to determine more than just age and growth. The chemical composition of otoliths can also reveal information about the life history of the fish. As the layers are formed on the otolith, the trace elements from the water the fish lived in, as well as the food the fish ate, bind with the calcium carbonate crystals. By examining the microchemistry of otoliths, scientists can study migration patterns, the unique habitats the fish lived in (especially the waterbodies the fish lived in and travelled through), diet, water temperatures, the presence of certain pollutants, and even more. Fisheries scientists can also match the chemical signature of the otoliths to the waterbody where the fish was born. For anadromous species (fish that live most of their lives in saltwater and migrate back into freshwater to spawn) such as salmon or river herring, the presence of elements such as strontium and barium can reveal when the fish first migrated into saltwater and when it eventually returned to spawn in freshwater. This is critical information for fisheries managers.
As trace elements bind to the calcium carbonate crystals during otolith formation, scientists can expose living fish to fluorescent chemicals, such as oxytetracycline and alizarin, to mark their otoliths. After the otolith is extracted from the fish, the marks can be seen using a fluorescence microscope (Figure 3). When viewed under fluorescent light, the marks appear as glowing rings (Figure 4). These chemical markings can be used to examine natal homing (if the fish returns to its birthplace to reproduce), age validation (to confirm the age of the fish), and the contribution of hatchery-reared fish to a wild population.
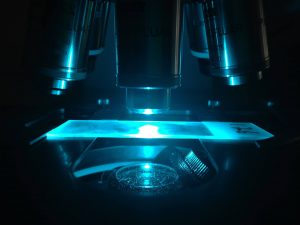
Figure 3. Examining an alewife otolith for oxytetracycline marks using a fluorescence microscope. Photo by Steve Luell.
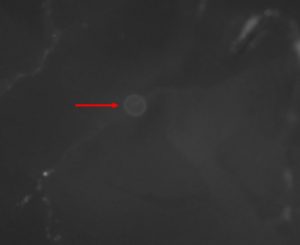
Figure 4. An oxytetracycline mark on the otolith of an alewife (from Luell 2016). Photo by Steve Luell.
Otoliths are an important tool for fisheries scientists around the world. They have applications far beyond just age and growth, they can be used for studying natal homing, migration patterns and even the habitats the fish lived in. By revealing vital information that is challenging to observe directly in fish, otoliths can contribute to effective fisheries management.
For more information on otoliths:
Bickford, N., and R. Hannigan. 2005. Stock identification of walleye via otolith chemistry in the Eleven Point River, Arkansas. North American Journal of Fisheries Management 25: 1542–1549.
Campana, S.E. 1999. Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188: 263-297.
Hendricks, M.L., R.L. Hoopes, D.A. Arnold, and M.L. Kaufmann. 2002. Homing of hatchery- reared American shad to the Lehigh River, a tributary of the Delaware River. North American Journal of Fisheries Management 22: 243-248.
Luell, S.M. 2016. Oxytetracycline and thermal marking of alewife (Alosa pseudoharengus) otoliths. MSc. thesis. University of New Hampshire.
Popper, A.N., J. Ramcharitar, and S.E. Campana. 2005. Why otoliths? Insights from inner ear physiology and fisheries biology. Marine and Freshwater Research 56: 497-504.
Rooker, J.R., D.H. Seco, G. DeMetrio, R. Schloesser, B.A. Block, and J.D. Neilson. 2008. Natal homing and connectivity in Atlantic bluefin tuna populations. Science 322(5902): 742- 744.